The Atriva Approach
The Atriva Approach
Atriva Therapeutics pursues a novel and unique approach
By combining antiviral and immunomodulatory activity via the downregulation of MEK in host cells, Atriva Therapeutics pursues a new approach for the development of treatments for severe RNA-virus induced infections in one tablet. Zapnometinib, the company`s lead product, has demonstrated a broadly active, efficacious and safe mechanism of action in pre-clinical and clinical studies. Atriva is therewith spearheading current therapeutic innovation in areas of high unmet medical need, such as severe influenza and other dangerous viral infections.
Zapnometinib (INN)
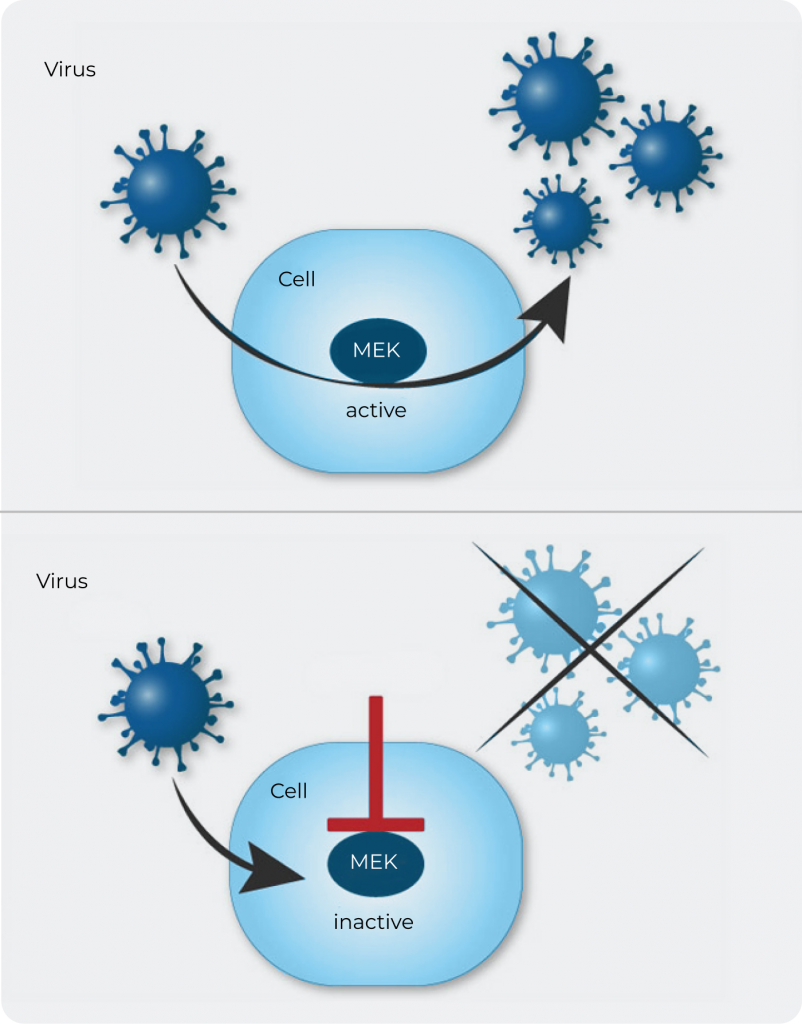
The company’s lead drug candidate zapnometinib targets the intracellular Raf/MEK/ERK signaling pathway. This pathway is highly relevant for the replication of many RNA viruses, such as the influenza virus, hanta virus or respiratory syncytial virus (RSV) and also SARS-CoV-2, the virus that causes COVID-19. In influenza virus infected cells, the interaction of zapnometinib with MEK (MAPK/ERK kinase) prevents export of the viral genome protein complexes (ribonucleoprotein, RNP) from the nucleus to the cytoplasm, thus blocking the formation of functional new viral particles. This ultimately reduces the viral load in the body.
Figure: Antiviral activity of zapnometinib: intracellular MEK inhibition.
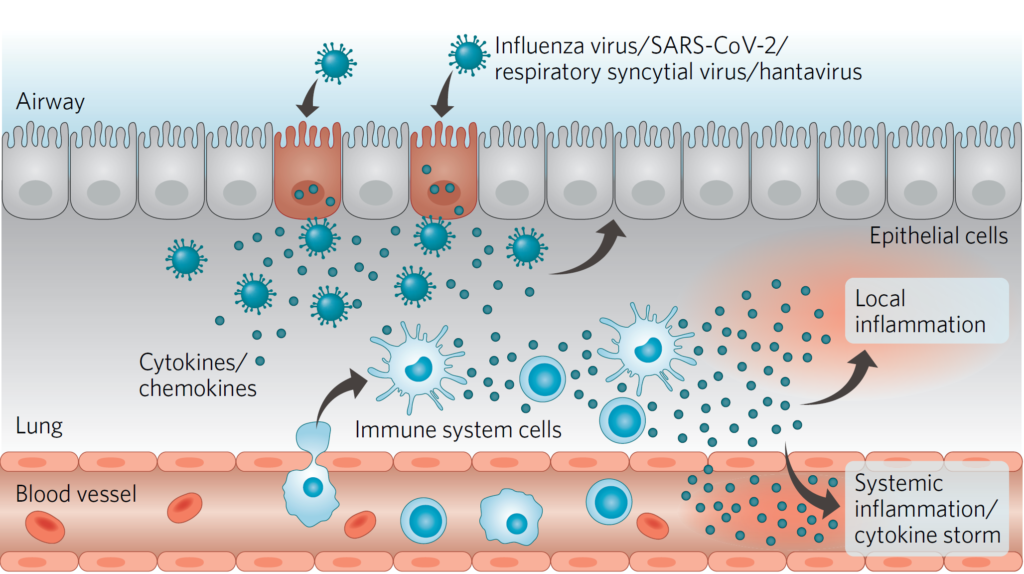
In addition, zapnometinib has the potential to modulate the pro-inflammatory cytokine response of the body caused by viral infections. Consequently, the drug can help avoiding an overshooting cytokine response and resulting complications. As demonstrated in vitro and in vivo by Atriva, MEK inhibition results in downregulated gene expression of some of the cytokines involved in exacerbating disease, like TNF-α, IL-1ß, IP-10, IL-8, MCP-1 and MIP-1a. Consequently the overactive inflammatory response can be mitigated in the lungs of patients who are severely ill with influenza or other severe RNA-virus-induced diseases.
Figure: Viral replication and cytokine storm. Zapnometinib addresses these effects, which often lead to severe progression of respiratory viral infections.
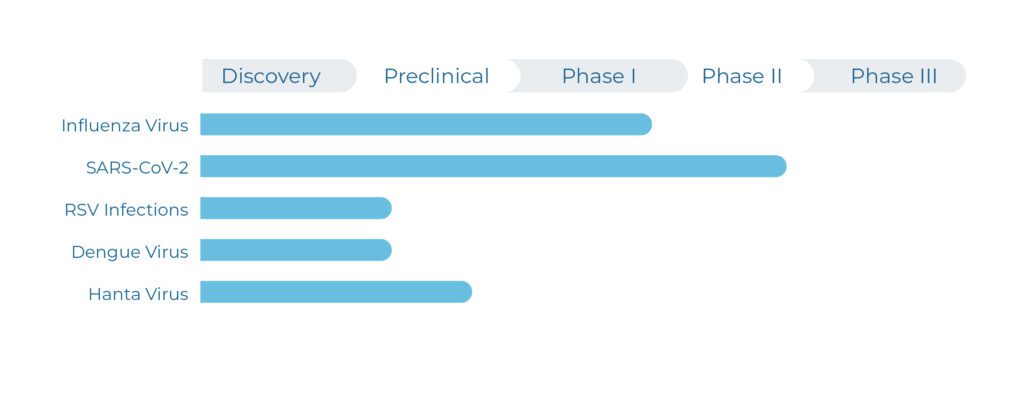
The company’s lead drug candidate is the MEK inhibitor zapnometinib. The small molecule, which can be administered orally (tablet), successfully completed Phase 2a development in COVID-19 and is in advanced clinical development in severe influenza, an indication with urgent need for effective and safe therapeutics.
In preclinical studies, Atriva has proven the antiviral efficacy of zapnometinib against various strains of influenza virus, hanta virus and respiratory syncytial virus (RSV). These promising data led to a Phase I clinical study with 70 healthy volunteers, which was successfully conducted in 2019 and demonstrated excellent safety and tolerability of zapnometinib (NCT04385420). In addition, pharmacokinetics exposure and determination of MEK inhibition were verified and confirmed the maintenance of clinically relevant blood levels. This supports the intended once-daily treatment regime for coming clinical development and subsequent commercialization.
Research & Development
The company’s lead drug candidate is the MEK inhibitor zapnometinib. The small molecule, which can be administered orally (tablet), is under advanced clinical development in two severe respiratory viral diseases: COVID-19 and influenza, two disease areas with urgent need for effective therapeutics.
Preclinical studies have proven the antiviral efficacy of zapnometinib against various strains of influenza virus, hantavirus and respiratory syncytial virus (RSV). These promising data led to a Phase I clinical study with 70 healthy volunteers, which was successfully conducted in 2019 and demonstrated excellent safety and tolerability of zapnometinib (NCT04385420). In addition, pharmacokinetics exposure and determination of MEK inhibition were verified and confirmed the maintenance of clinically relevant blood levels. This supports the intended once-daily regime for the ongoing Phase II clinical development.
Discover the ongoing clinical trials in COVID-19 and influenza.
Pipeline
The company’s lead drug candidate is the MEK inhibitor zapnometinib. The small molecule, which can be administered orally, is under advanced clinical development in severe influenza and completed a clinical trial in COVID-19. In the area of severe influenza where there are currently no therapeutic options, there is an urgent need for effective and safe therapeutics.
As a first-in-class treatment for severe respiratory viral infections, including influenza, that use the Raf/MEK/ERK signaling pathway in host cells, zapnometinib is also studied in haemorrhagic viral infections, such as hantavirus or dengue virus, and provides a strong platform portfolio.